What is ISO 15189 Accreditation?
ISO 15189:2012 specifies quality management and technical requirements for medical laboratories. Accreditation demonstrates that your lab produces reliable, clinically valid results through competent personnel, validated methods, effective QC/EQA, and robust information management.
Achieving ISO 15189 builds trust among clinicians, patients, regulators, and payers—supporting patient safety and timely clinical decisions.

- Safer patient care through validated methods and result assurance
- Higher clinician confidence and improved diagnostic utility
- Stronger QMS discipline and risk-based oversight
- Reduced repeats, errors, and TAT variability
- Better compliance with regulatory and payer requirements
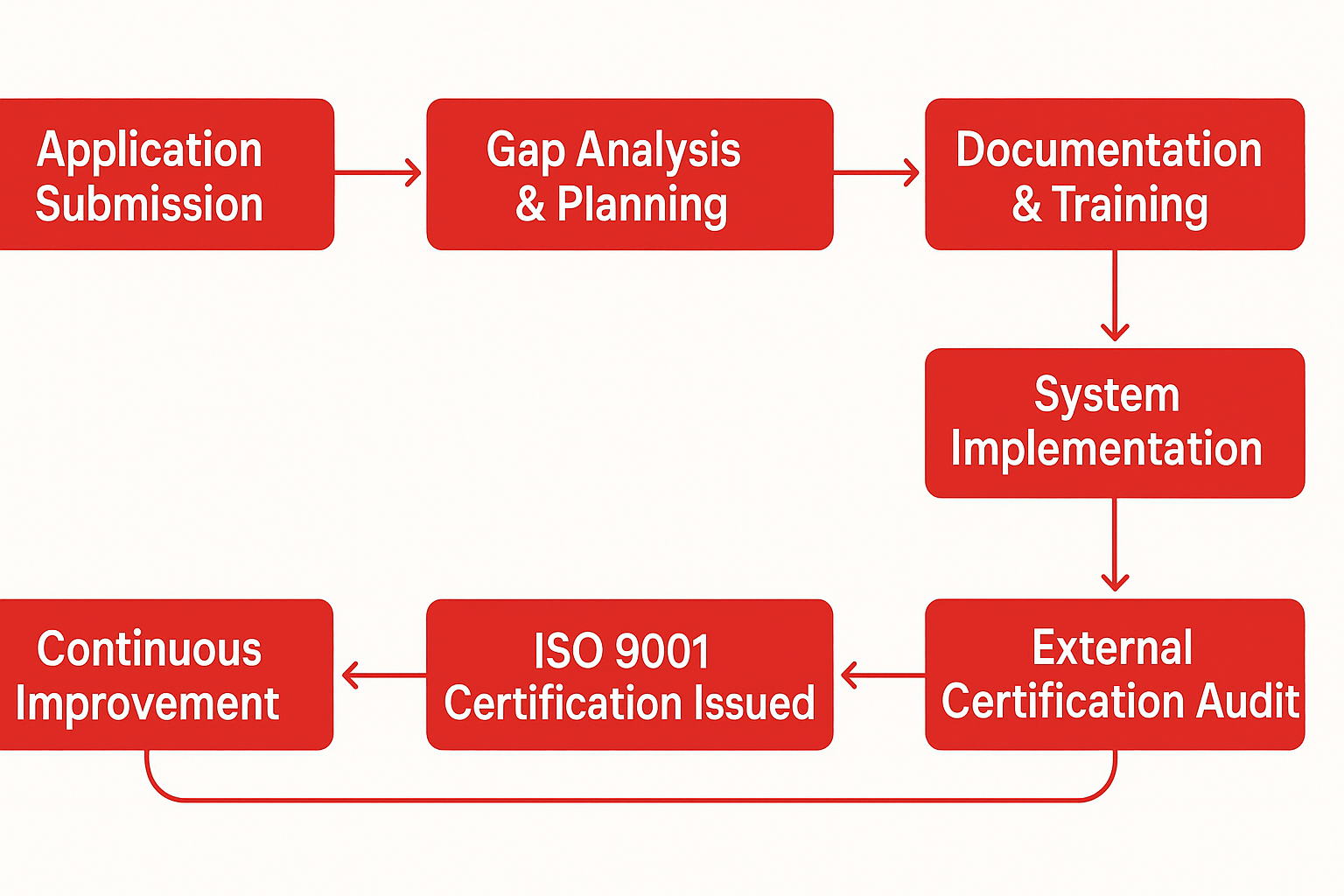
ISO 15189 Accreditation Process
- Application & Scope – Disciplines, sites, test menu
- Gap Analysis & Plan – Timelines and responsibilities
- Documentation & Training – QMS, SOPs, competence
- Implementation – Method V/V, IQC/EQA, equipment controls
- Internal Audit & Management Review – Readiness check
- Assessment – AB Stage 1 document review and on-site witnessing
- Corrective Actions – NCR closure and evidence
- Accreditation Decision – Certificate issuance
- Surveillance – Periodic visits and re-assessment
Why Choose Us?
- Deep bench of assessors/consultants with clinical lab backgrounds
- Ready-to-use templates for V/V, IQC, EQA/PT, and LIS controls
- Hands-on training and competency frameworks
- Experience with multi-site and POCT oversight models
- Practical, audit-ready implementation support
Why Get 15189 Now?
- Increasing quality expectations from hospitals and payers
- Supports network tie-ups and public health participation
- Reduces risk and improves clinician satisfaction
- Drives measurable improvement in patient-centric outcomes


