Overview
Good Distribution Practice (GDP) is a set of guidelines for the proper distribution of pharmaceutical products. It ensures that the medicines are stored, transported, and handled in a way that maintains their quality, safety, and efficacy.
Achieving GDP certification is a necessity for pharmaceutical distributors, enabling compliance with international standards and improving reliability in distribution.

- Storage Requirements – Secure, temperature-controlled, and clean environments.
- Transportation Standards – Maintaining product integrity during transportation.
- Traceability – Record-keeping for effective tracking and product recall.
- Staff Training – Ensuring personnel are trained in GDP guidelines and practices.
- Regulatory Compliance – Adherence to national and international distribution standards.
GDP Certification Process
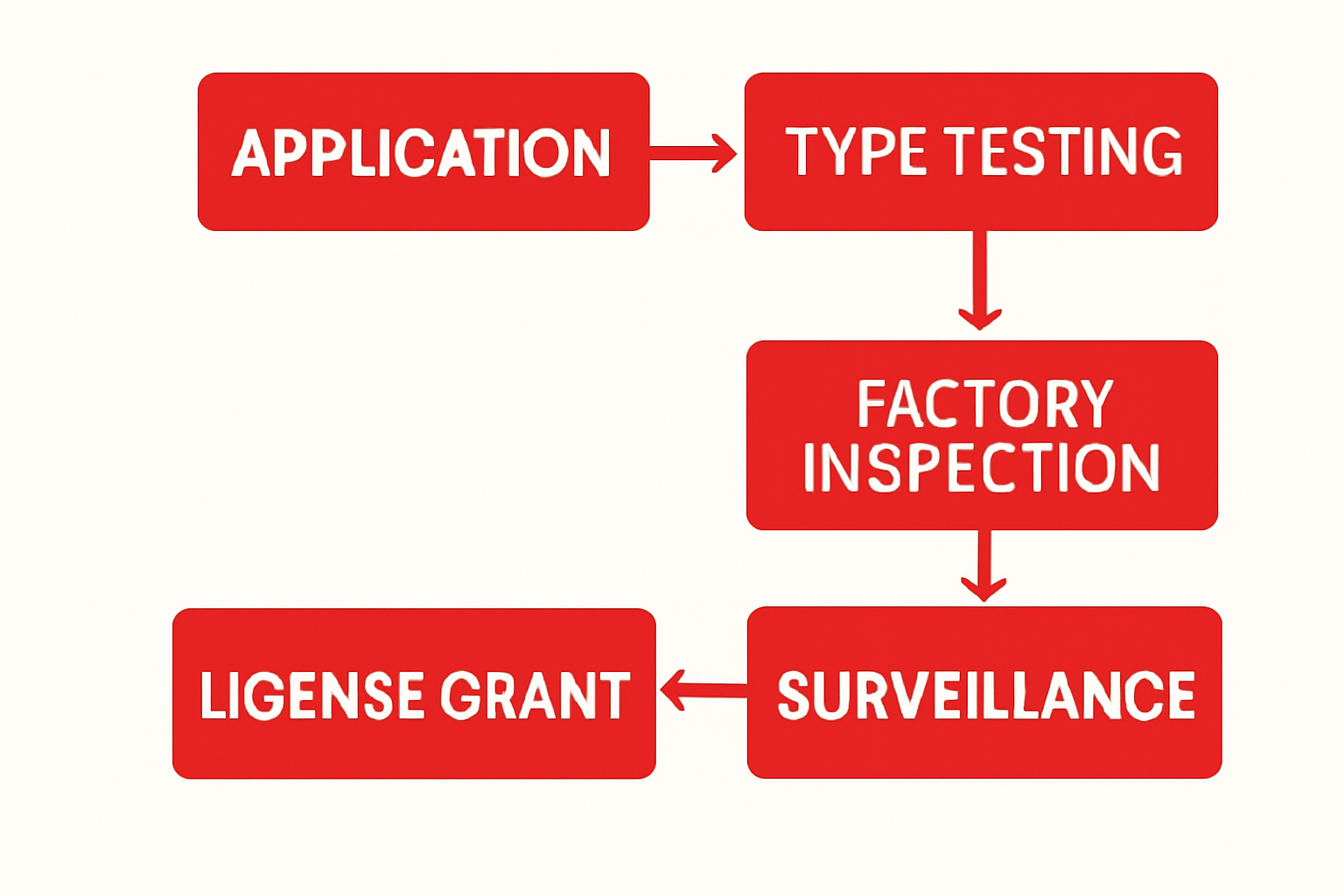
- Application – Submit company details and product list.
- Inspection – GDP auditors inspect storage and distribution practices.
- Product Testing – Verification of temperature, packaging, and shelf life standards.
- Certification – Grant of GDP certification after passing audits and tests.
- Surveillance – Ongoing monitoring and compliance checks.
Why Choose Us?
- Experienced consultants with expertise in pharmaceutical distribution compliance.
- End-to-end service from application to certification.
- Assistance with inspection preparation and documentation.
- Compliance guidance for regulatory bodies.
- Single-window solution for all certification needs.
Our Services
- GDP Certification (Good Distribution Practice)
- ISO Certification (9001, 14001, 45001)
- Regulatory compliance consulting
- Supply chain risk assessment and mitigation
- Packaging and storage compliance audits


